In Context
One in eight women will be diagnosed with breast cancer during their lifetime. Early detection significantly improves patient outcomes, making breast cancer the most treatable cancer. Despite this, breast cancer remains the leading cause of cancer-related deaths among women.
This is why women aged 50 and above are routinely referred to the hospital for mammograms every two years.
The Blue Box is developing an In Vitro Diagnostic device for non-invasive, early detection of breast cancer through a urine sample.
This device will initially complement mammogram-based screening when an inconclusive result is obtained, prior to performing a biopsy.
However, with an expected Negative Predictive Value of 99.7%, compared to 95.6% for mammograms, The Blue Box could become the new standard of care for breast cancer screening.
The Challenge

Perdigó's Approach
Perdigó always demonstrates great flexibility and profound commitment when working with ambitious and fast-moving start-up clients like The Blue Box.
From a redesign of the device architecture and Industrial Design to the development of customised embedded electronics and compliant firmware, the development of The Blue Box has been a truly multidisciplinary project.
Some highlights of this In Vitro Diagnostic device engineering include:
Device concept: The Blue Box device has a clean, beautiful and user-friendly design housing its advanced technology.
Firmware compliance: medical products that integrate software that is crucial for its functionality must be developed under ‘Software in Medical Devices’ (SiMD) standards. For instance, ISO 62304 defines software life cycle requirements while ISO 81001 is focussed on cybersecurity.


Physics simulations: Perdigó has performed both numerical and analytical analyses of the dynamic heating of the urine sample. This has accelerated the development of this core technology of The Blue Box device.
Electromagnetic compatibility: ensuring that The Blue Box is robust against electromagnetic interference and does not emit electromagnetic disturbances that may affect other equipment in the laboratory.
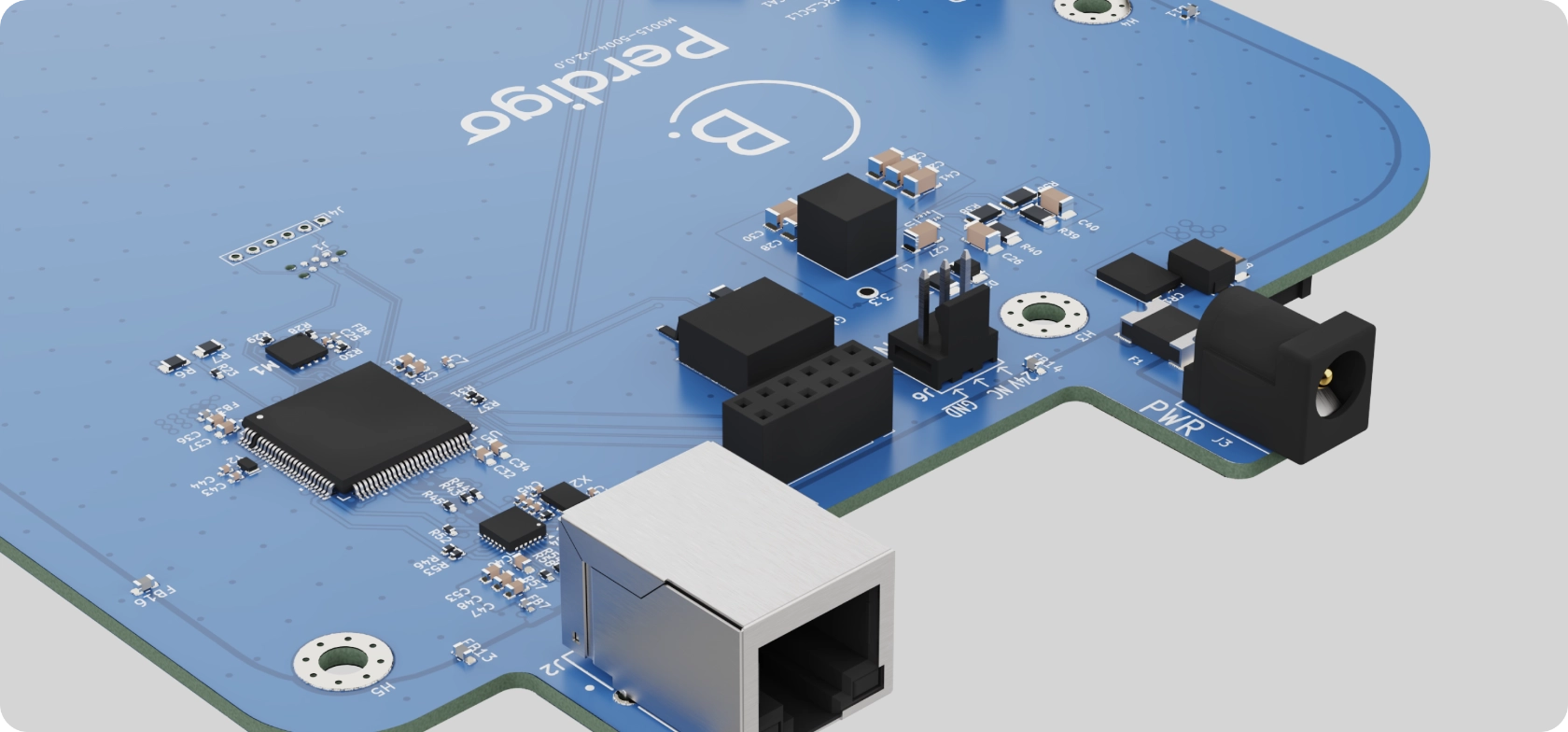
Ethernet connectivity: with the main device interface in the web-application, a robust internet connection is essential for The Blue Box. Somewhat counter-intuitively, a wired connection is the most user-friendly option in the device’s use environment.
Transfer to manufacturing: Perdigó has identified and successfully worked with manufacturing partners for The Blue Box that have greatly reduced time to market, technical risk and cost.
Delivered Value
The result is a beautifully designed and well engineered device: from precise sample heating to advanced sensor technology and connectivity features. The first dozens of The Blue Box devices have been produced to conduct a clinical trial, demonstrating a breakthrough in breast cancer screening. In parallel, the Technical Documentation is supporting the device’s CE marking.
