
Electronics Engineering
Hardware development
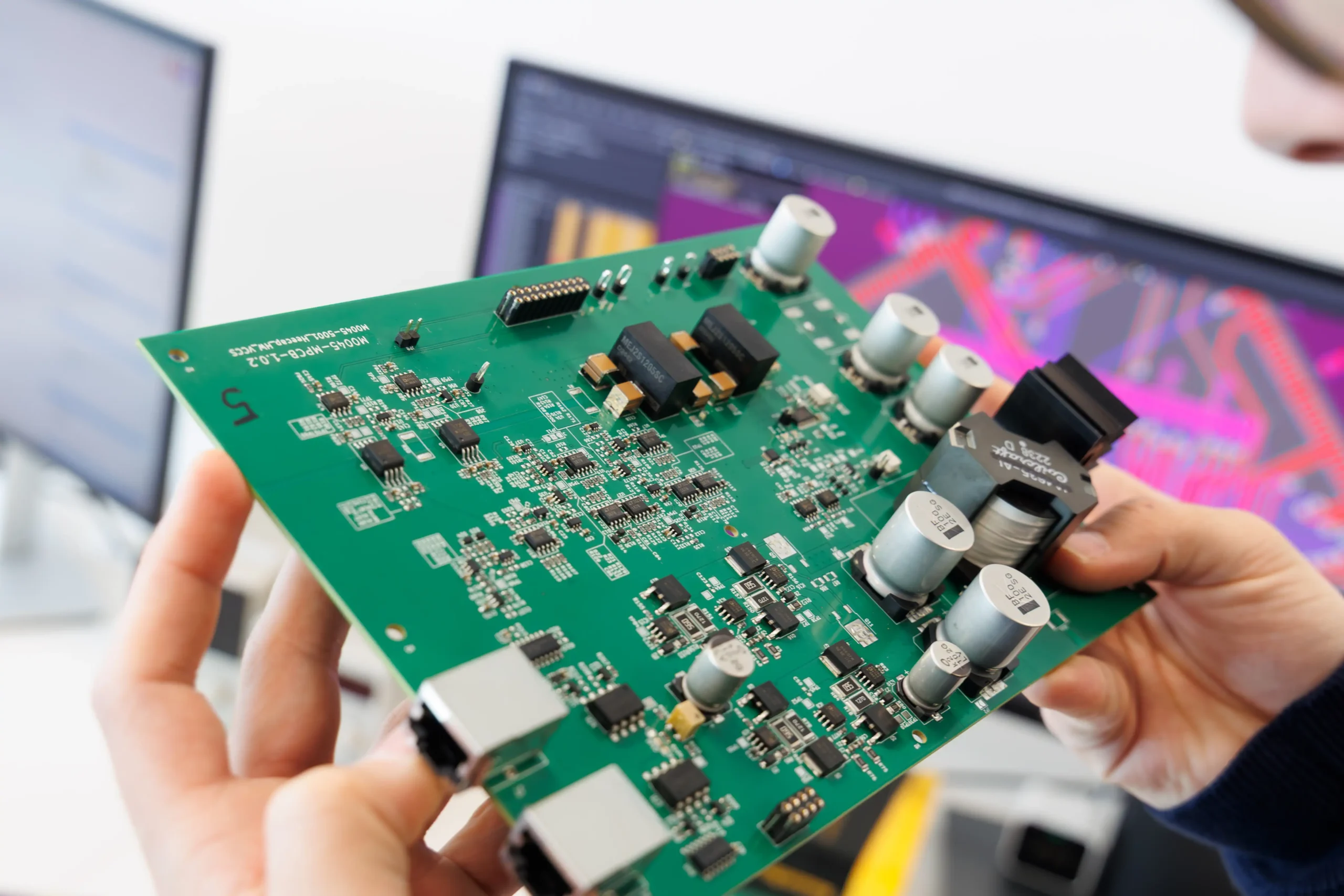
Robust, high-performance electronic hardware is tailored to the specific requirements of medical devices.
All stages are covered, from conceptualisation and simulation to PCB layout, design, component selection, and miniaturisation. Every aspect is meticulously engineered to ensure reliability, safety, and longevity.

Firmware development
The team excels in developing customised embedded firmware and advanced control systems, delivering tailored solutions from low-level software to comprehensive automated control.
Expertise ensures seamless integration and optimised performance across a wide range of applications, from microcontroller-based devices to sophisticated automation systems.
Integrated Systems Design
Hardware and firmware are brought together in seamless integration, ensuring that devices operate flawlessly. Experience with various microcontrollers, sensors, and communication protocols enables the creation of systems that are both innovative and reliable.

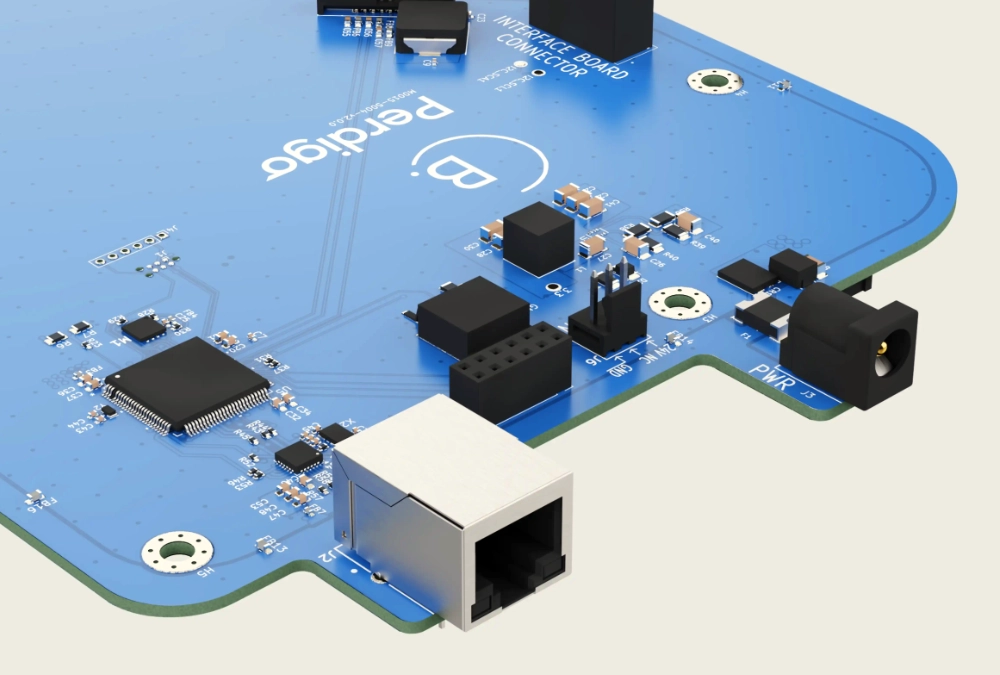
Connectivity Solutions
Hardware and firmware are seamlessly integrated, ensuring that devices operate flawlessly. Experience with various microcontrollers, sensors, and communication protocols enables the creation of systems that are both innovative and reliable.
Comprehensive hardware design services are offered, including the integration of BLE, NFC, and Ethernet technologies. This approach ensures reliable and efficient connectivity solutions tailored to specific needs, whether for wireless communication, secure data transfer, or robust networking.
Regulatory Compliance
Designs are created with compliance in mind, minimising the time and cost associated with regulatory approvals.
The firmware development process is documented in accordance with IEC 62304. To achieve this, Doxygen is integrated into the software development environment to document the code and maintain comprehensive and organised documentation.

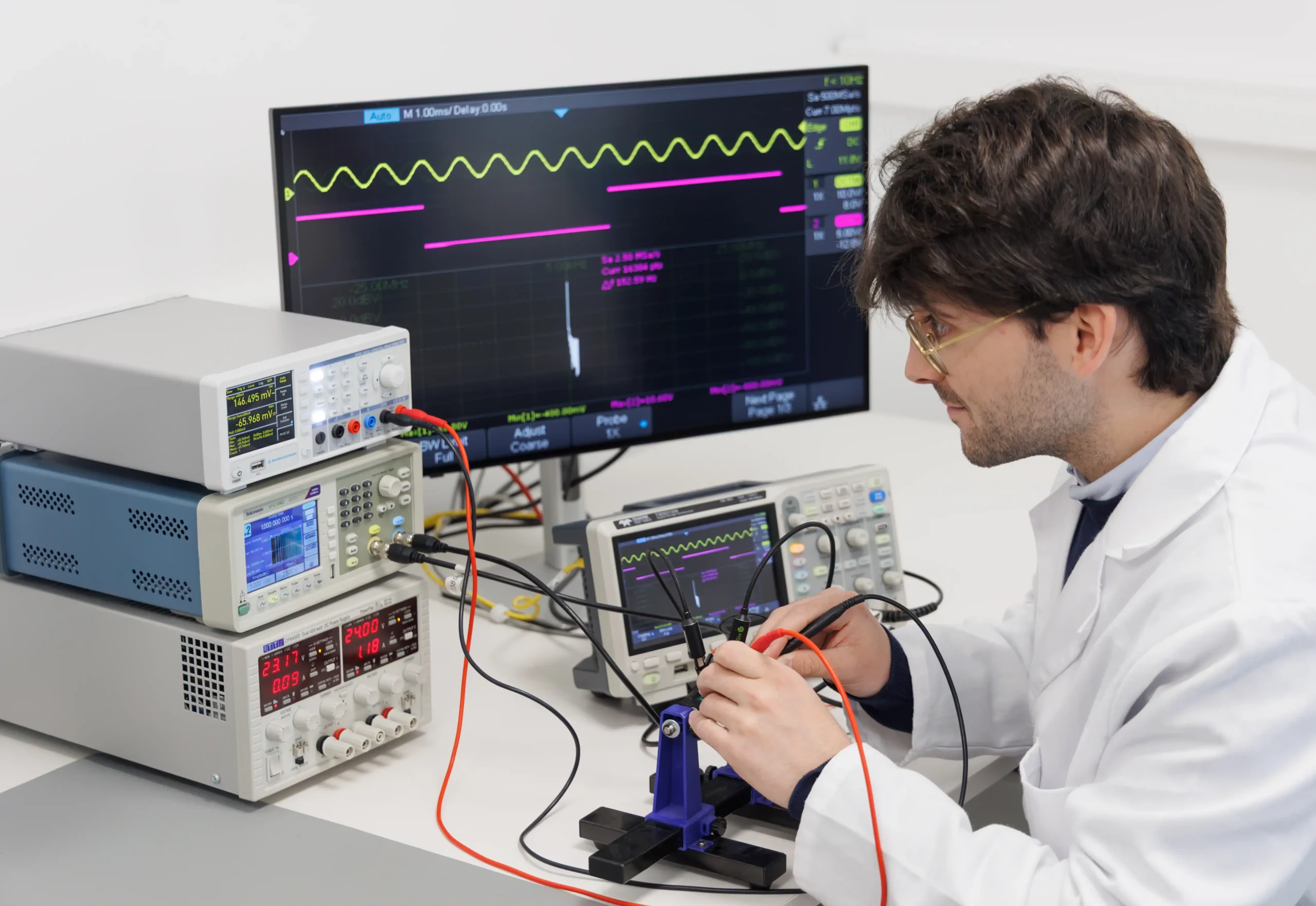
Prototyping and Testing
Rapid prototyping, along with rigorous testing and debugging, is at the core of the development process. The working methodology relies on testing the hardware and firmware at every stage, ensuring that medical devices operate seamlessly and safely in critical environments.
Preliminary testing helps identify potential issues early, reducing the risk of costly redesigns and ensuring compliance with standards like IEC 60601.